Can my compensation control be too bright?
No. In fact, the brighter, the better! Compensation controls allow the software to calculate how much spillover fluorescence occurs from that level of primary fluorescence. The equation calculates the ratio of the DIFFERENCE in the secondary fluorescence over the DIFFERENCE in primary fluorescence (essentially, a slope). Therefore, any fluorescence that falls within that range is compensated correctly.
Remember, the spillover value is a percent. Whether the primary fluorescence is 10,000 or 100, using the percent spillover, the software accurately calculates the amount of secondary fluorescence.
Probably around 90% of compensation issues actually arise due to the use of insufficiently bright controls. If your sample stains brighter than your control, the software cannot accurately calculate the amount of secondary fluorescence and will overcompensate or under compensate the data.
For more information, see the controls section of this document.
Can I use compensation particles to compensate cells?
Yes. The medium used for compensation is irrelevant as long as the positive and negative control for any given parameter have the same autofluorescence. Since you are calculating the DIFFERENCE in fluorescence between the positive and negative, if they have the same autofluorescence, then autofluorescence becomes 0. In fact, that is what we want! We don’t want autofluorescence as any part of our compensation calculation, as it will negatively affect the precision of the calculation.
If the autofluorescence of your positive is 10 fluorescent units and the autofluorescence of your negative is 10 fluorescent units, then when you subtract the negative from the positive, autofluorescence is now 0.
What if my compensation value is over 100%?
It doesn’t really matter. We could try and explain this here, but the post on the New England Cytometry blog does a great job summing it up!
Can I create an empty matrix to type in my own values?
Yes, but you shouldn’t! Compensation is only as accurate as your single stain controls. Creating your own matrix is dangerous and subjective. Remember, there is a saying in the flow community that, “There are the biggest lies of lies and then there is flow cytometry!” Lets not propagate that any more!
Ok, ok… time to get off the soap box and show you how to do it (even though you shouldn’t).
1. Double click on the little white box (empty matrix) next to a file in the workspace:

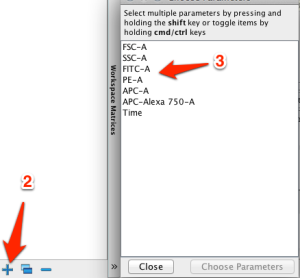
2. Click the plus (+) sign in the matrix list and
3. select the parameters to use in the matrix:
An empty matrix will be created:
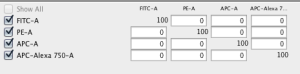
You can edit the values and use the [M] button to drag and drop the matrix to a sample or a group of samples.
How do I edit an existing matrix?
Open the matrix editor. Click the edit button on the compensation matrix you want to edit. This will duplicate the matrix and allow you to edit the matrix. Use the [M] button to drag and drop the matrix to a sample or a group of samples.
